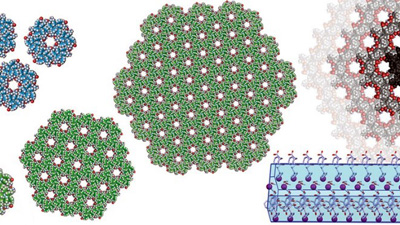
Slavko Kralj from the Department for Materials Synthesis with co-authors from Italy (Silvia Marchesan and others) published an article in ACS Nano Heterochirality and Halogenation Control Phe-Phe Hierarchical Assembly. By analogy with the current pandemic situation, the authors tried to understand the impact of “social distancing” at the nano level. The peptide diphenyl alanine (Phe-Phe) is an important basic building block of amyloid structures. The homochiral Phe-Phe forms toxic amyloid aggregates due to intermolecular hydrophobic (social) interactions. The authors found that the heterochiral Phe-Phe forms intramolecular (asocial) hydrophobic interactions, which, however, prevent hierarchical bundling into more complex anisotropic structures. The authors therefore showed that supramolecular nanostructures formed by self-assembly of heterochiral Phe-Phe do not exhibit amyloid toxicity to cells. The research thus reveals the importance of heterochirality of short peptides in supramolecular chemistry, which is an important basis for understanding peptide aggregation and amyloid formation.”