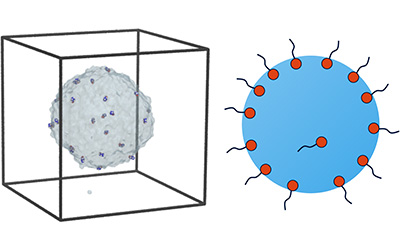
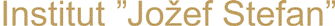Researchers Fabio Staniscia and Matej Kanduč from the Department of Theoretical Physics used computer simulations to reveal that surface-active molecules (surfactants) bind differntly to highly curved water interfaces—such as those found on nanometer-sized droplets and bubbles—compared to flat surfaces. This discovery challenges the long-standing assumption that interfacial behavior is independent of system size and offers important new insights into the behavior of colloidal systems at the nanoscale. Surfactants are among the most widely used chemical substances globally, present in cleaning products, cosmetics, pharmaceuticals, food formulations, and many industrial applications. A better understanding of surfactant behavior at curved interfaces allows for more precise formulation of emulsions, foams, and other colloidal systems, and contributes to improved modeling of environmental phenomena like aerosol behavior and cloud formation. The study was published in the renowned Journal of Colloid and Interface Science and highlights how fundamental physical discoveries can shape our understanding and have far-reaching implications for science and technology.