More about the Institute |
|
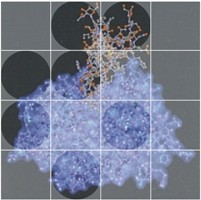
Evolvement of research areas The ability to isolate and purify proteinases enabled several 3D structures to be determined in collaboration with the Max Planck Institute in Munich. With the advent of recombinant DNA technology, it became possible to study the structure of the genes for proteinases, to make mutant proteins in order to explore their mechanism of action, and to produce enzymes, present in very small amounts in tissues, in quantities sufficient for structural studies. It therefore became appropriate for the Department to acquire its own X-ray crystallographic facility in 1995, and already the structures of cathepsins, alone and in combination with their specific inhibitors, are helping to explain the intricate molecular basis for controlling the potentially destructive activity of proteinases in vivo. Another of the intriguing findings to emerge from this crystallographic structure work concerns evolutionary economy shown in the papain family of proteinases. Those enzymes whose structures have so far been studied employ a common workhorse structure to cleave the peptide bond of their substrate proteins. However, the biologically important specificity for cleavage exhibited by individual members has evolved by subtle changes in the surface topology and, in some, by the addition of structural loops or peptides. |
J. Stefan Institute, Jamova 39, 1000 Ljubljana, Slovenia, Telephone: +386 1 477 39 00 |